About Green Fluorescent Protein
Green Fluorescent Protein (GFP) has existed for more than one hundred and sixty million years in one species of jellyfish. Green fluorescent protein acts as a reporter of expression, which can be used in the observation of protein expression.
The green fluorescent protein (GFP) is a protein from the jellyfish Aequorea victoria that fluoresces green when exposed to blue light. This process takes place when the protein aequorin, also produced by A. victoria, interacts with Ca2+ ions thus emitting a blue glow.
GFP may be used to study the dynamics of many processes within the cell. These include protein folding, protein transport, and RNA dynamics.
In a wide range of pH, increasing pH leads to a reduction in fluorescence by 395 nm excitation and an increased sensitivity to 475 nm excitation. Hence GFP will express its florescent phenotype when exposed to ultra violet rays. Thus its presence can be observed.
How to incorporate GFP into E. coli?
One possible way is to transform Escherichia coli with the pGLO plasmid using the heat-shock method.
The transformed and non-transformed cells are grown on separate LB plates with/without ampicilin and arabinose. The transformed and non-transformed bacterial colonies on different plates are then compared with the positive control plate without ampicilin. Then a bacterial colony taken from an LB plate with ampicilin and arabinose is inoculated in a 50 ml liquid culture and these bacteria are used for GFP purification. GFP is then purified from bacterial proteins using chromatography of hydrophobic interactions, which is a three-step process, each step demanding a special buffer.
Overall Objective
The objective of our experiment is to produce green fluorescent protein by culturing genetically modified E. coli, which carry Green fluorescent protein gene, in a bioreactor.
DAY 1
To familiarize with the parts and components of microbial bioreactors.
To learn the basic operation procedure of a bioreactor.
DAY 2
To learn the steps to prepare a bioreactor
To prepare the media for seed culture and scale-up fermentation
To prepare the seed culture for scale-up fermentation
DAY 3
To carry out scale-up fermentation process to increase the yield of desired protein product (Green florescent protein in this case)
To monitor cell growth and product formation through manual sampling and computer data logging
DAY ONE!
Equipment, Media and Seed Culture Preparation
Today was the start of the first Bioprocess Technology Practical. We gathered at room Q604 where the empty bioreactors were placed in front of us. To start off, we were given about 15mins to study the bioreactor and hence identify its components. With the help of the diagrams and chart in the practical manual (pg 7&8) we simply filled in as many blanks as possible. Soon, Mr Ong Chee Ming, our demonstrator for the practical, came back to check on our progress =D. He was amazed that we actually knew what rotameter is for =P. Below shows the diagram of what we had filled in ;)
Here is how the actual bioreactor in our lab looks like...
The functions of various parts of the fermentor:
Besides learning about the purpose of the various parts of a bioreactor, we also discovered that different colored tubes connected to the bioreactor in and out are used for various functions. This is to enable easy recognition of the parts of the bioreactor. The table below illustrates the different colored tubes for various parts of the bioreactor.
Our co-demonstrator, Meng Chuan showed us how to do sampling. After which we were all given a chance to practise sampling, with water in the vessel.
Below are the summarized steps of how sampling is done:
1. Unclamp coupling point at the fermentor
2. Pull syringe completely to suck
3. Push syringe completely to remove excess back to the fermentor
4. Clamp coupling point at the fermentor
5. Unplug syringe, pull syringe completely, attached syringe back
6. Unclamp coupling point at the collection port
7. Push syringe completely to transfer sample into test tube
8. Clamp coupling point at the collection port
Media Preparation
After being briefed on the parts of the bioreactor, we went to the next room to prepare the media. The room stank badly; Mr Ong said it was due to the yeast. Our job was to prepare 2L of LB(Luria-Bertani) media which comprised of 10g Bacto-tryptone, 5g Yeast extract and 10g NaCl per litre. Fortunately for us, all of these were already mixed in a single container and all we had to do was dissolve 25g of the LB powder into 1L of water twice since the maximum volume of water which the beaker could hold was only a litre. The LB powder to be dissolved was weighed using a balance and it was transferred into a 1L beaker.
At the same time, some of us used a measuring cylinder to measure 500mL (max. capacity) of distilled water. The 25g of powder was first added to the 1L beaker followed by the 500mL of water. We then measured another 500mL of water and filled the beaker to the brim. The next step involves dissolving the LB powder completely. This was done with the help of a magnetic stirrer.
It was rather interesting to watch the vortex of powder swirling round as the magnetic stirrer was activated. We repeated the same steps another time thereafter to obtain the final 2L of LB media required which was subsequently transferred a big glass flask which we carried back to the room with the bioreactors to be briefed on the preparation steps.
Transferring the freshly-made LB broth into a flask
Bioreactor Preparation
Upon entering the room and gathering around the 2nd bioreactor, Meng Chuan told us that most of this part of the practical had been done for us and that we would simply be briefed on the preparations steps to be made to the bioreactor.
We were told that the pH probe had to be calibrated before each use and that we had to make sure that the foam probe was above the liquid level while the level probe just touching the media surface. As the foam rises, it causes an electrical discharge within the foam probe which then connects a circuit which releases antifoam into the broth culture, thereby reducing the foam level.
Other things we noted was that of pre-sterilization steps where all cables(with the exception of the temperature probe) had to be disconnected, all silicone tubes clamped(except for exhaust filter and female STT coupling of sampling unit) and also to cover all filters and sockets with aluminum foil to protect against any condensing moisture.
Preparation of seed culture
Firstly, we streaked a LB/Amp/Ara plate with colonies of pGLO transformed E.coli, after which we left it to incubate for 24hrs.
DAY TWO!
Checking on the Growth of Cells
We placed the agar plate of E. coli on a UV lamp to check if it contained pGLO which encodes the GFP gene. The colonies were glowing!
Now we can proceed with inoculating the flask containing 100mL LB medium with Ampicillin with the E. coli. The culture gave a clear yellow color. Meng chuan mentioned that it will become cloudy the next day; due to growth of the E. coli. The flask was then left to incubate for 1 day.
Kah Hong inoculating the E. coli!
DAY THREE!
Inoculation, Fermentation and Monitoring
For scale-up fermentation, we let the medium broth cool to below 50degreeC before adding Ampicillin to a final concentration of 100ug/ml. We then added arabinose to a final concentration of 0.2%. With the control parameters set as shown below, we inoculated the fermentor with 100ml of seed culture [5% of fermentation medium volume]. Fermentation was allowed to continue for 24hrs at the conditions listed below.
The cloudy culture!
Monitoring the conditions of the fermentor
We took a 10ml blank sample before inoculation and subsequently a sample of 10ml was taken every hour. This is to monitor the fermentation process. After 24hrs of fermentation, the broth was harvested. 10ml of culture was then transferred into a sterile, disposable test tube.
Here are our results!
below is our Cell Growth Plot...
what does it tell us?
From the graph, we can see that cell concentration is steadily increasing over time for the first 5 hours. The trend shown in the graph should be in the exponential phase as the cell have already adapt to the media and fermenter conditions with its enzymes needed for cell metabolism have already been synthesizes and activated through cofactors and thus begin to proliferate quickly in the medium. Around 6 hours later, we observed that the batch culture is entering the stationary phase as we can see the graph forming a straight line. The cells are not growing very quickly at this stage. The number of cells growing is almost equivalent to the number of cells dying.
our glowing pellet!
DAY FOUR!
Isolation and Purification of Product
Stage 1: Isolation
Green Fluorescent Protein (GFP) is an intracellular product, hence the bacteria cells need to be lysed first to release the protein. Three methods of cell disruption will be performed on our bacteria cells.
We first obtain the cells by centrifugation at 10,000 rpm for 5 minutes. This step helps to separate the cells from the liquid broth. The cells are denser and so form a pellet at the bottom of the tube. The liquid broth is less dense and constitutes the supernatant. Pour the supernatant into another tube. The product can be confirmed to be in the pellet by observing both tubes under UV light.
Method 1: Using Enzymes
We then re-suspend the pellet in 500ul of TE buffer of pH 7.5 using a micropipettor until there are no visible clumps. Using a disposable 1mL plastic pipette, two drops of lysosome were added to the re-suspended cell pellet. This will initiate the enzymatic digestion of the bacteria cell wall. The enzymes were allowed to act for 15 minutes.
Method 2: Freezing and Thawing
We placed the tube in liquid nitrogen until the contents are frozen. Next, the tube was thawed in warm water. The cycle of freezing and thawing was repeated (for another 2 times) to complete the rupturing of the bacteria cell wall. Freezing and thawing add mechanical stress to the cell wall as the cell water content expands (when frozen) and contracts (when thawed).

whoa it's freezing!

thawing.
Method 3: Sonication
The cell disruption is completed by the process of sonication where ultrasonic waves (of higher frequency than sound) cause the bacteria cell wall to implode under the vibrational pressure. Sonication is done on ice for 4 cycles of 25 seconds with 10 seconds rest in between sonication cycles.

After cell disruption, we spun the contents of the tube in a centrifuge for 20 minutes at 10,000 rpm. The pellet and supernatant were then separated as done previously. We then re-suspended the pellet using 400ul of TE buffer.
The GFP product will now be found in the supernatant!
Stage 2: Purification
We obtained the extract from stage 1 and purified it using size exclusion chromatography/gel permeation. This method of purification uses a column of a polymer gel resins (Sephadex G75). The resins contained very small pores in which molecules that are small enough can diffuse within. After which, we poured the extract into the column, where the larger molecules will flow through the column faster and the smaller molecules will spend more time interacting and diffusing into the pores of the gel resins. Using this concept, we achieved separation of the different molecules by size.
Procedure
1. We labeled eight test tubes (from 1 to 8) and a blank, which are then placed on a rack.
2. The blank tube was filled with 2.0ml of ammonium bicarbonate and we used this tube as a guide to mark the remaining test tubes with a line at the 2.0ml level.
3. We carefully drained the column into a waste beaker until the buffer is just even with the top of the gel bed. We took extra precaution so as to not let the column run dry.
4. Using a disposable glass pipette, we transferred the cell-free extract to the top of the gel bed by gently swirling the pipette around the inside edge of the column, just above the top of the packed matrix.
5. We took fractions filled in each test tube by removing the waste beaker and placing the test tube under the stopcock. We then filled each test tube to the 2.0ml mark.
6. Slowly, we opened the stopcock to allow the sample to flow completely into the gel bed, collecting the eluting buffer in our first test tube. Again, we took extra caution to not let the column run dry by adding buffer.
7. 50mM of ammonium bicarbonate buffer was added to the top of the column whilst we were taking the fractions. The buffer was maintained at a 2-3cm mark above the gel column to provide a consistent flow of buffer through the chromatography matrix.
8. We continued filling fractions of 2ml each until the 8th tube was filled up.
Stage 3: AnalysisWe took absorbance readings using the spectrophotometer set at 476nm, which is the wavelength at which GFP strongly absorbs and gives out its usual fluorescence.
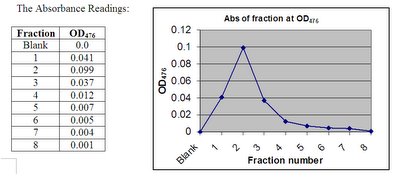
We used ammonium bicarbonate as a blank, and absorbance readings of the fractions were taken using the spectrophotometer and a graph of the readings was plotted against the fractions. From the graph, we can see that fraction 2 gave a peak at which the highest absorbance reading was achieved, which means that the fraction contained the most GFP, allowing a high amount of fluorescence to be absorbed. Another reason is that the larger molecules are eluted first in size exclusion chromatography. Therefore fractions 1, 2 and 3 had higher readings as they are able to absorb a high amount of fluorescence. There was then a gradual decrease in the subsequent fractions, as most the GFP were eluted in the earlier fractions (fractions 1, 2 and 3), and that these fractions contained smaller molecules as smaller molecules are eluted last in size exclusion chromatography. The last two fractions had very small absorbance readings of 0.004 and 0.001, which is almost zero, therefore we conclude that all the GFP have been eluted and are collected in the 8 tubes. Thus our sample is quite pure as it was well separated from other unwanted proteins.
What we have learnt:
1. Fermentation is not just about breaking up complex substances into simple substances; it is about producing the desired product.
2. Cells are modified to produce the desired product.
3. It is important to understand the parts and components of the equipments involve in the fermentation process, and hence are able to perform precise operations through the process.
4. The choice of cells to use is essential, as it determines the end-product produced, the amount produced, as well as it compatibility to sustain the process with the available equipments.
5. Technology has advanced over time such that monitoring of the process throughout the process is computerized. This provides convenience in bioprocess field.
Familiarization with the Bioreactor and its Operation
Q1)State the differences you observe between a microbial bioreactor and a mammalian cell bioreactor.
In a microbial bioreactor, both impeller and bubble column device can be installed. Whereas in a mammalian cell bioreactor, only bubble column can be installed. This is so as mammalian cell are very fragile cells, and would be damaged upon the strong propelling force of the impeller.
Q2)Study the work flow on page 1 of your laboratory manual. Describe the typical activities that are performed for each stage in the fermentation process.
Familiarization with the Bioreactor and its Operation (Exp 1)
In this stage, parts and functions of the bioreactor is being introduced and elaborated on. This is to ensure that the operators are clear about operations to carry upon with a bioreactor, such as sampling, throughout the fermentation process.
Equipment, Media and Seed Culture Preparation (Exp 2)
This is the stage where by the input of the fermentation process is being prepared. Equipment has to be ensured sterility and set in the correct operations, media prepared with exact proportion, and that the culture to be seeded is genetically modified to produce the desired product. All in all ensuring that these 3 key factors : Equipment, Media and seed culture, to commence the fermention process to be carried out to be a success generate the desired product.
Inoculation, Fermentation and Monitoring (Exp 3)
During this stage, accurate analyze of the entire process is crucial. This is by inoculating sample consistently (hourly), thus monitoring the progress inside the bioreactor tank. By doing so, graphs can be plot and hence operators are able to deduce the process of the fermentation taking place in the bioreactor. If any point of time the fermentation goes wrong, the operator would be able to make necessary changes to factors such as pH and temperature, so as to divert the fermentation back on track.
Isolation and Purification of Product (Exp 4)
Final stage of a fermentation process is be isolate the desired products out of the bioreactor tank. This can be done through dilution, column chromatography and sonification, and filtering, so as to purify the desired product and thus obtained it from the fermentation process.
Equipment, media and seed preparation
Q1) Media Preparation
a. Explain the purpose of each ingredient found in the LB media.
Bacto-tryptone – Provides essential amino acids needed for E.coli growth
Yeast extract – Provides vitamins and certain trace elements
NaCl – Provides sodium ions for transport and osmotic balance
Water – To dissolve the LB powder and provide an aqueous environment for
E.coli to thrive
b. What is the purpose of ampicillin?
The purpose of adding ampicillin is to screen out any contaminating bacteria as well as to select for only E.coli which have been transformed presumably successfully with the gfp gene which was encoded together with the same plasmid containing the gene for ampicillin resistance(bla gene).
c. Why is ampicillin added only after autoclaving?
Ampicillin is added only after autoclaving as the high heat(121oC for 20min) involved would disrupt the structure of the functional group(beta lactam ring) involved in its antimicrobial activity, rendering the antibiotic useless.
Q2) Equipment Preparation
a. What is meant by calibration of the pH probe?
Calibration of the pH probe involves immersing it into at least 2 buffers, 1 with a pH of 7 and another to match the desired range of pH being measured. The meter is adjusted until it measures the correct pH in the 2 solutions. This makes the probe accurate in measuring pH within that range.
b. Why is Hydrochloric acid not suitable as a correction agent for pH?
HCl is a strong acid and so does not make a good buffer. Usually, a weak acid and a conjugate base is used.
c. What is meant by polarisation of the pO2 probe?
Probe polarization is essential for stable measurements with the same recurring degree of accuracy. With the probe properly polarized, oxygen is continually used up by passing through the sensitive diaphragm and dissolving in the electrolyte solution contained within the probe. If this operation is interrupted, the electrolyte solution continues to be enriched with oxygen until it reaches an equilibrium with the surrounding solution.
Whenever measurements are taken with a non-polarized probe, the oxygen level revealed is both that of the tested solution as well as that present in the electrolyte solution, which therefore results in an incorrect reading being taken.
d. What is a peristaltic pump?
A peristaltic pump is a positive displacement pump that is used for pumping fluids. It works in a peristaltic motion much like the muscle walls of the gastrointestinal tract and is often used to maintain the sterile conditions of the fluid in the bioprocess context.
Q3) Seed Preparation
a. What is the purpose of arabinose?
Arabinose provides an alternative carbon source to glucose for E.coli to metabolize.
b. Describe the sterile techniques used in seed preparation.
Hands were sprayed with ethanol prior to work done which is all within the fume hood.
c. Why do we perform step-wise scale-up instead of tranferring directly to the fermentor?
Conditions within the fermenter is much different than that of a lab scale culture. As such, factors such as aeration, distribution of nutrients must be taken into consideration. Step-wise scale up allows for us to monitor how the organisms adapt to the changing environment better and to adjust the parameters accordingly to cater to the organisms growth.
Inoculation, Fermentation and Monitoring
Q1) Explain the control philosophy for pH, temperature and dissolved oxygen as was used in the fermentation process.
The pH probe measures pH of the culture broth in real time. This information is fed into the main computer control system which provides a negative
feedback mechanism such that any fluctuation in pH is corrected in order to stabilize the pH at the value set. This correction is done by the addition of
appropriate amounts of acid/alkali/buffer through the use of an external containment unit connected to pumps and interfaced with the main computer control system.
The maintenance of a constant temperature and dissolved oxygen content works in a similar way, with the temperature probe and dissolved oxygen probe measuring
their respective parameters and the main computer control providing the negative feedback calculations to allow for the amount of oxygen to be sparged into the
culture broth or the increase/decrease in the rate of flow of cooling water within the cooling jacket.
























